GLP-1's Really Work

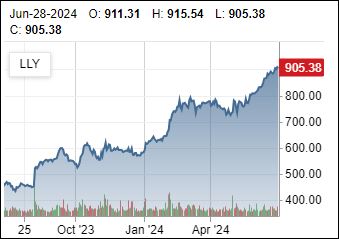
Novo hints at price negotiations
Explore the Rapidly Evolving Field of RealWtLoss Drugs

Starting Your Journey
Step 1: Commit to a Change in Life Style
Overall 10-20% of individuals stop GLP-1 Meds due to side effects (Nausea, Vomiting, Diarrhea, Constipation, Pancreatitis (rare), Other) Go Slow! Consider not increasing the Dose and let the body adjust. Going the next Dose too soon can result in overwhelming symptoms.
20-40% of individuals who complete a course of GLP-1 Meds regain their weight
Step 2: Meet FDA Criteria For
Diabetes Type 2
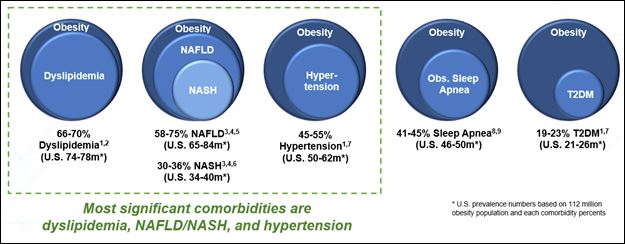
Obesity
Obesity and CV Disease
Obesity: BMI 30 or Greater
Diabetes Type 2: Elevated PSA
Obesity and Cardiovascular Disease: BMI > 27 with Cardiovascular Disease
Step 3: Get A Prescription



Suggestions: Don’t Goit Alone ! Beware Non FDA Approved Injectables !
Step 4: Get The Meds (not so easy!)

Novo Nordisk (Wegovy and Ozempic) as well as Eli Liily (Mounjaro and Zepbound) are unable to keep up with demand. It is not uncommon to make multiple calls in your area to check on availability. Direct to consumer is now possible with Amazon, Lilly Direct, WW and others
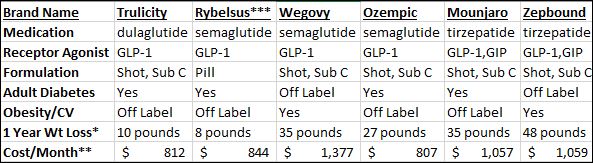
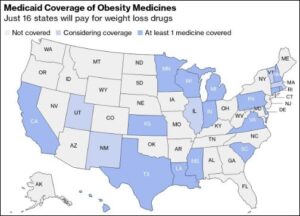
Cost of Your Journey



The Future: Pipeline of GLP-1 Drugs
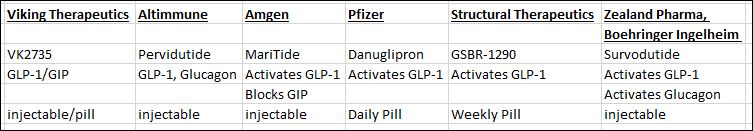
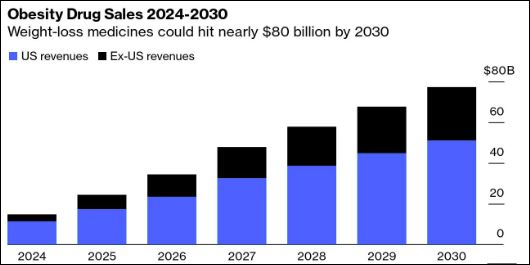
Billion Dollar Opportunity
In The Lead
Head and Neck
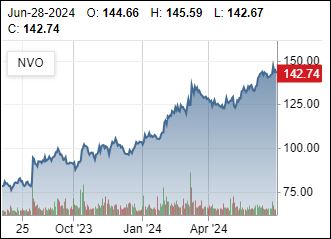
NASH Data?

Pill vs Shot?
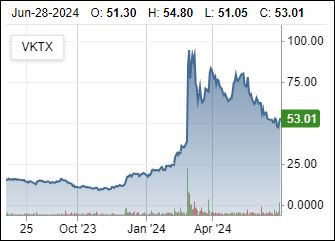
Demand Dictates Room For More
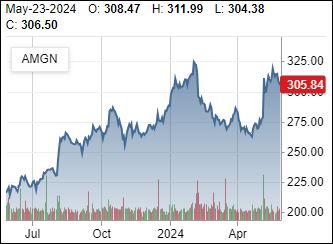
Mimics GLP-1)
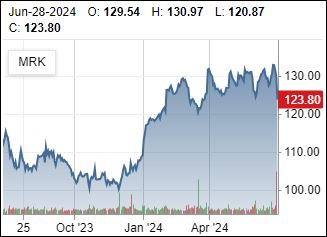
GLP-1 News

10/2/2024: Compounding pharmacies are suing the Food and Drug Administration so they can keep making imitation versions of popular—and lucrative—tirzepatide drugs, namely knockoffs of Mounjaro for diabetes and Zepbound for weight loss.
10/1/2024: FDA announces tirzepatide no longer in short supply, potentially shutting down generic compounding.
9/15/2024: Novo appears to be ready to negotiate pricing
9/1/2024: Zepbound 2.5-mg vial ( 1 month $399) or 5 mg vial ($549) direct to consumer from Lilly.
8/20/2024: A three-year study of tirzepatide – a medication approved in the US as Mounjaro for diabetes and Zepbound for weight loss – found that when adults who had prediabetes and obesity or overweight used it weekly, it lowered their risk of progression to diabetes by 94% compared with a placebo, according to drugmaker Eli Lilly.
7/30/2024: Patients with type 2 diabetes taking semaglutide (Ozempic) had fewer tobacco-related medical encounters and smoking cessation interventions compared with those on other diabetes drug.
7/29/2024: Swiss pharmaceutical giant Roche said Monday it is accelerating the development of its Wegovy rival weight loss drugs following promising early stage trial data.
7/25/2024 : Weight loss drugmakers Eli Lilly (NYSE:LLY) and Novo Nordisk (NVO) were among the notable decliners in the premarket Thursday after Viking Therapeutics (NASDAQ:VKTX) announced plans to start a late-stage trial for its obesity candidate VK2735 later this year
7/17/2024: Roche said its experimental once-daily pill CT-996 resulted in a placebo-adjusted average weight loss of 6.1% within four weeks in obese patients without Type 2 diabetes in a Phase I trial
7/12/2024: Ozempic linked with lower dementia risk, nicotine use, British study finds. Ongoing studies with Ozempic and Alzheimer's.
7/10/2024: Pfizer announced it will move forward with a once-daily version of its weight loss pill, danuglipron, after it saw “encouraging” data in an ongoing early-stage study.
7/5/2024: Researchers identified a possible link between semaglutide and a rare eye condition but said more research is needed to know whether the drug actually caused the problem.
6/25/2024: In the words of Altimmune's CEO, Vipin Garg, the trial's results (of weight loss and muscle sparing) suggest that pemvidutide could be a "best in class" medicine if it ultimately gets approved.
6/21/2024: Lilly's tirzepatide reduced obstructive sleep apnea (OSA) severity, with up to 51.5% of participants meeting the criteria for disease resolution
6/10/2024: A phase 2 trial involving participants with MASH and moderate or severe fibrosis, treatment with tirzepatide for 52 weeks was more effective than placebo with respect to resolution of MASH without worsening of fibrosis.
5/21/2024: More than one-half of patients with Crohn's disease treated with Lilly's mirikizumab achieved clinical remission at one year, including patients with previous biologic failure (further raising the value of Lilly stock).
5/2/2024: Amgen will no longer develop an early-stage obesity pill, and will instead focus on a more advanced injectable candidate that’s seen as a potential competitor to Novo Nordisk’s Wegovy and Eli Lilly’s Zepbound.
4/20/2024: A judge dismissed Lilly claims accusing an online pharmacy of selling an unauthorised version of its diabetes drug Mounjaro (tirzepatide)
4/17/2024: The first drug to treat fatty liver disease due to metabolic dysfunction-associated steatohepatitis (MASH) has been given a green light by the US Food and Drug Administration. Madrigal Pharmaceuticals’ Rezdiffra (resmetirom) received an accelerated approval to treat the disease, previously known as non-alcoholic steatohepatitis (NASH).
4/6/2024: Among patients with obesity-related heart failure with preserved ejection fraction and type 2 diabetes, semaglutide led to larger reductions in heart failure–related symptoms and physical limitations and greater weight loss than placebo at 1 year. (DOI: 10.1056/NEJMoa2313917)
4/2/2024: Costco will offer weight-loss program to members through medical partner. The service, which will cost $179 every three months, is scheduled to become available April 2.
3/26/2024: Viking Phase 1 results, which are supposed to test the safety of the drug, showed up to 5.3% weight loss in patients after 28 days. The pill showed no safety issues and a majority of side effects were mild — a key differentiator from current market injectable leaders
3/14/2024: Madrigal Pharmaceuticals’ resmetirom, to be sold under the brand name Rezdiffra, has become the first MASH therapy to clear the FDA finish line. Viking Therapeutics has VK2809, which, like Rezdiffra, is an oral thyroid receptor-beta agonist, still being tested. Lilly in February reported positive phase 2 MASH data for its GIP/GLP-1 dual agonist tirzepatide in overweight or obese people, although the study wasn’t statistically powered to measure improvement in fibrosis.
3/8/2024: Wegovy can now be used to reduce the risk of stroke, heart attacks and other serious cardiovascular problems in patients who are overweight or who have obesity, the FDA said. This opens the door for Medicare Part D coverage.
10/1/2024: FDA announces tirzepatide no longer in short supply, potentially shutting down generic compounding.
9/15/2024: Novo appears to be ready to negotiate pricing
9/1/2024: Zepbound 2.5-mg vial ( 1 month $399) or 5 mg vial ($549) direct to consumer from Lilly.
8/20/2024: A three-year study of tirzepatide – a medication approved in the US as Mounjaro for diabetes and Zepbound for weight loss – found that when adults who had prediabetes and obesity or overweight used it weekly, it lowered their risk of progression to diabetes by 94% compared with a placebo, according to drugmaker Eli Lilly.
7/30/2024: Patients with type 2 diabetes taking semaglutide (Ozempic) had fewer tobacco-related medical encounters and smoking cessation interventions compared with those on other diabetes drug.
7/29/2024: Swiss pharmaceutical giant Roche said Monday it is accelerating the development of its Wegovy rival weight loss drugs following promising early stage trial data.
7/25/2024 : Weight loss drugmakers Eli Lilly (NYSE:LLY) and Novo Nordisk (NVO) were among the notable decliners in the premarket Thursday after Viking Therapeutics (NASDAQ:VKTX) announced plans to start a late-stage trial for its obesity candidate VK2735 later this year
7/17/2024: Roche said its experimental once-daily pill CT-996 resulted in a placebo-adjusted average weight loss of 6.1% within four weeks in obese patients without Type 2 diabetes in a Phase I trial
7/12/2024: Ozempic linked with lower dementia risk, nicotine use, British study finds. Ongoing studies with Ozempic and Alzheimer's.
7/10/2024: Pfizer announced it will move forward with a once-daily version of its weight loss pill, danuglipron, after it saw “encouraging” data in an ongoing early-stage study.
7/5/2024: Researchers identified a possible link between semaglutide and a rare eye condition but said more research is needed to know whether the drug actually caused the problem.
6/25/2024: In the words of Altimmune's CEO, Vipin Garg, the trial's results (of weight loss and muscle sparing) suggest that pemvidutide could be a "best in class" medicine if it ultimately gets approved.
6/21/2024: Lilly's tirzepatide reduced obstructive sleep apnea (OSA) severity, with up to 51.5% of participants meeting the criteria for disease resolution
6/10/2024: A phase 2 trial involving participants with MASH and moderate or severe fibrosis, treatment with tirzepatide for 52 weeks was more effective than placebo with respect to resolution of MASH without worsening of fibrosis.
5/21/2024: More than one-half of patients with Crohn's disease treated with Lilly's mirikizumab achieved clinical remission at one year, including patients with previous biologic failure (further raising the value of Lilly stock).
5/2/2024: Amgen will no longer develop an early-stage obesity pill, and will instead focus on a more advanced injectable candidate that’s seen as a potential competitor to Novo Nordisk’s Wegovy and Eli Lilly’s Zepbound.
4/20/2024: A judge dismissed Lilly claims accusing an online pharmacy of selling an unauthorised version of its diabetes drug Mounjaro (tirzepatide)
4/17/2024: The first drug to treat fatty liver disease due to metabolic dysfunction-associated steatohepatitis (MASH) has been given a green light by the US Food and Drug Administration. Madrigal Pharmaceuticals’ Rezdiffra (resmetirom) received an accelerated approval to treat the disease, previously known as non-alcoholic steatohepatitis (NASH).
4/6/2024: Among patients with obesity-related heart failure with preserved ejection fraction and type 2 diabetes, semaglutide led to larger reductions in heart failure–related symptoms and physical limitations and greater weight loss than placebo at 1 year. (DOI: 10.1056/NEJMoa2313917)
4/2/2024: Costco will offer weight-loss program to members through medical partner. The service, which will cost $179 every three months, is scheduled to become available April 2.
3/26/2024: Viking Phase 1 results, which are supposed to test the safety of the drug, showed up to 5.3% weight loss in patients after 28 days. The pill showed no safety issues and a majority of side effects were mild — a key differentiator from current market injectable leaders
3/14/2024: Madrigal Pharmaceuticals’ resmetirom, to be sold under the brand name Rezdiffra, has become the first MASH therapy to clear the FDA finish line. Viking Therapeutics has VK2809, which, like Rezdiffra, is an oral thyroid receptor-beta agonist, still being tested. Lilly in February reported positive phase 2 MASH data for its GIP/GLP-1 dual agonist tirzepatide in overweight or obese people, although the study wasn’t statistically powered to measure improvement in fibrosis.
3/8/2024: Wegovy can now be used to reduce the risk of stroke, heart attacks and other serious cardiovascular problems in patients who are overweight or who have obesity, the FDA said. This opens the door for Medicare Part D coverage.
Share Your Experience
Email us at: contact@realwtloss.com