GLP-1 Drug History
Dulaglutide (Trulicity) Exenatide (Byetta) Exenatide Extended Release (Bydureon BCise) Liraglutide (Victoza) Lixisenatide (Adlyxin) Semaglutide subcutaneous, tablet (Ozempic, Wegovy, Rybelsus) Tirzepatide (Mounjaro, Zepbound)
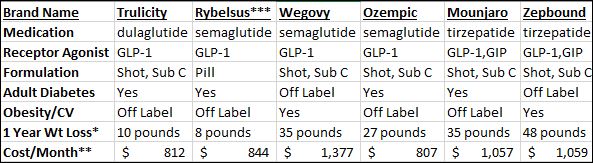
*Data varied per med dose, duration of treatment.
** Cost estimate 11/2023 without insurance. Check Drug Websites for discount programs.
***Rybelsus must be taken by mouth on an empty stomach when you first wake up with a sip of plain water (no more than 4 ounces).
- Do not split, crush, or chew. Swallow RYBELSUS whole
- After 30 minutes, you can eat, drink, or take other oral medicines
Mounjaro Compared To Wegovy/Ozempic
Data Published by Eli Lilly
In a 40-week study, the Mounjaro 5-mg, 10-mg, and 15-mg doses were compared to Ozempic 1 mg in 1879 adults with type 2 diabetes who were also taking metformin and had a starting A1C of 8.3%.
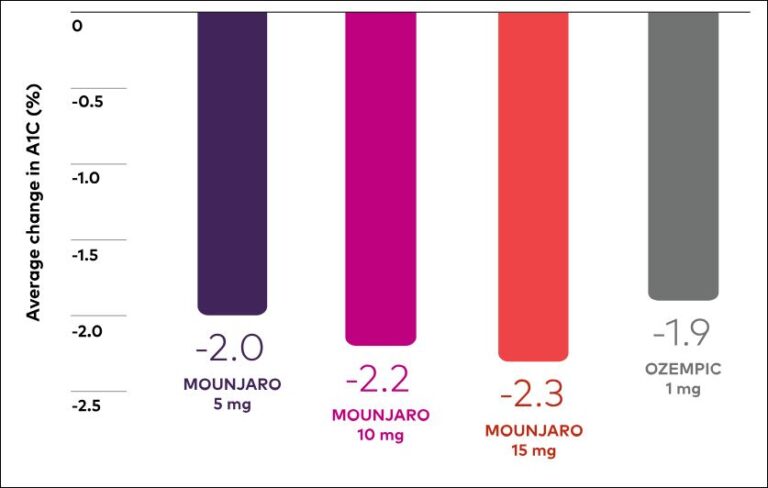
Mounjaro vs Ozempic (1MG) Weight Loss
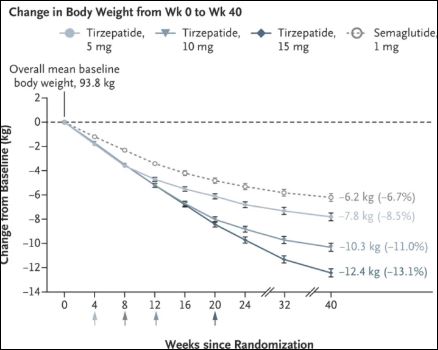
Second Nature: Oct 2023
Mounjaro/Zepbound
Mounjaro/Zepbound (tirzepatide) is a dual glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptor agonist (RA) that was recently approved by the FDA for the treatment of type 2 diabetes. The drug is manufactured by Eli Lilly & Co. under the brand name Mounjaro™ and was approved in May 2022 and in Nov 2023 Zepbound for weight loss (with associated comorbidities) .
Physicians can prescribe Mounjaro OFF LAbel for Weight Loss. Zepbound is approved for Weight Loss and associated comorbidities.
Tirzepatide Contraindications
Tirzepatide is contraindicated in patients with a personal or family history of MTC or in patients with Multiple Endocrine Neoplasia syndrome type2 . Counsel patients regarding the potential risk of MTC and symptoms of thyroid tumors. Tirzepatide causes thyroid C-cell tumors in rats. It is unknown whether Mounjaro causes thyroid C-cell tumors, including medullary thyroid carcinoma (MTC), in humans as the human relevance of induced rodent thyroid C-cell tumors has not been determined Tirzep
Tirzepatide Warnings and Precautions
Pancreatitis: Has been reported in clinical trials. Discontinue promptly if pancreatitis is suspected.
Hypoglycemia with Concomitant Use of Insulin Secretagogues or Insulin: Concomitant use with an insulin secretagogue or insulin may increase the risk of hypoglycemia, including severe hypoglycemia. Reducing dose of insulin secretagogue or insulin may be necessary.
Hypersensitivity Reactions: Hypersensitivity reactions have been reported. Discontinue tirzepatide if suspected.
Acute Kidney Injury: Monitor renal function in patients with renal impairment reporting severe adverse gastrointestinal reactions.
Severe Gastrointestinal Disease: Use may be associated with gastrointestinal adverse reactions, sometimes severe. Has not been studied in patients with severe gastrointestinal disease and is not recommended in these patients.
Diabetic Retinopathy Complications in Patients with a History of Diabetic Retinopathy: Has not been studied in patients with non-proliferative diabetic retinopathy requiring acute therapy, proliferative diabetic retinopathy, or diabetic macular edema. Monitor patients with a history of diabetic retinopathy for progression.
Acute Gallbladder Disease: Has occurred in clinical trials. If cholelithiasis is suspected, gallbladder studies and clinical follow-up are indicated
Tirzepatide can increase your heart rate while you are at rest.
Tirzepatide Adverse Reactions
The most common adverse reactions, reported in ≥5% of patients treated with tirzepatide are: nausea, diarrhea, decreased appetite, vomiting, constipation, dyspepsia, and abdominal pain.
Similar to Ozempic and Wegovy
Tirzepatide: Pregnancy
Pregnancy: Based on animal study, may cause fetal harm.
Females of Reproductive Potential: Advise females using oral contraceptives to switch to a non-oral contraceptive method, or add a barrier method of contraception for 4 weeks after initiation and for 4 weeks after each dose escalation
Tirzepatide: Injectable
Tirzepatide is a once a weekly injectable prescription medicine that is used along with diet and exercise to improve blood sugar (glucose) in adults with type 2 diabetes mellitus. The FDA approved Zepbound for weight loss.
Mounjaro has different dose options, allowing you and your doctor to find the one that’s right for you. If your doctor has prescribed once-weekly Mounjaro, you’ll start with 2.5 mg. After 4 weeks, you’ll move on to 5 mg.
In studies with or without other diabetes medications, 75% to 90% of patients reached an A1C of less than 7%, with an average starting A1C of 7.9% to 8.6% across the 5-mg, 10-mg, and 15-mg doses
Wegovy/Ozempic
Wegovy/Ozempic/Rybelsus are semaglutides. Wegovy is simply a higher dose Ozempic. Semaglutides are glucagon-like peptide-1 receptor agonist, or GLP-1, drugs. All three of these are made by Danish pharmaceutical company Novo Nordisk. Ozempic and Rybelsus are indicated for type 2 diabetes, whereas Wegovy is specifically indicated for the treatment of overweight and obesity.
Semaglutide Contraindications
Semaglutide is contraindicated in patients with a personal or family history of MTC or in patients with Multiple Endocrine Neoplasia syndrome type2 . Counsel patients regarding the potential risk of MTC and symptoms of thyroid tumors. Semaglutide causes thyroid C-cell tumors in rats. It is unknown whether Mounjaro causes thyroid C-cell tumors, including medullary thyroid carcinoma (MTC), in humans as the human relevance of induced rodent thyroid C-cell tumors has not been determined
Semaglutide Warnings and Precautions
Pancreatitis: Has been reported in clinical trials. Discontinue promptly if pancreatitis is suspected.
Hypoglycemia with Concomitant Use of Insulin Secretagogues or Insulin: Concomitant use with an insulin secretagogue or insulin may increase the risk of hypoglycemia, including severe hypoglycemia. Reducing dose of insulin secretagogue or insulin may be necessary.
Hypersensitivity Reactions: Hypersensitivity reactions have been reported. Discontinue MOUNJARO if suspected.
Acute Kidney Injury: Monitor renal function in patients with renal impairment reporting severe adverse gastrointestinal reactions.
Severe Gastrointestinal Disease: Use may be associated with gastrointestinal adverse reactions, sometimes severe. Has not been studied in patients with severe gastrointestinal disease and is not recommended in these patients.
Diabetic Retinopathy Complications in Patients with a History of Diabetic Retinopathy: Has not been studied in patients with non-proliferative diabetic retinopathy requiring acute therapy, proliferative diabetic retinopathy, or diabetic macular edema. Monitor patients with a history of diabetic retinopathy for progression.
Acute Gallbladder Disease: Has occurred in clinical trials. If cholelithiasis is suspected, gallbladder studies and clinical follow-up are indicated.
Wegovy can increase your heart rate while you are at rest.
Semaglutide Adverse Reactions
The most common side effects of Wegovy™ may include: nausea, diarrhea, vomiting, constipation, stomach (abdomen) pain, headache, tiredness (fatigue), upset stomach, dizziness, feeling bloated, belching, gas, stomach flu and heartburn.
FDA Warning: Potential for intestinal obstruction
Semaglutide: Pregnancy
Pregnancy: Based on animal study, may cause fetal harm.
Females of Reproductive Potential: Advise females using oral contraceptives to switch to a non-oral contraceptive method, or add a barrier method of contraception for 4 weeks after initiation and for 4 weeks after each dose escalation
Wegovy/Ozempic: Injectable
Wegovy™ (semaglutide) is a weekly injectable prescription medicine used for adults with obesity (BMI ≥30) or overweight (excess weight) (BMI ≥27) who also have weight-related medical problems to help them lose weight and keep the weight off.
Ozempic® (semaglutide) injection 0.5 mg, 1 mg, or 2 mg is a once-weekly injectable prescription medicine medicine for adults used to improve blood sugar, along with diet and exercise, and reduce the risk of major cardiovascular events such as heart attack, stroke, or death in adults with type 2 diabetes and known heart disease.
Wegovy is simply a higher dose than Ozempic.

